Thankfully, cases of transformers catching fire are rare. A major North American utility reported an overall failure rate of 1.21 percent per year on their entire fleet of 765 kV transformers over a 20-year period [1]; only 0.14 percent of these involved fires.
When fires do occur, the consequences can be dramatic. The highly flammable insulating fluid found in many transformers can feed a fire for a long time and the smoke may contain toxic substances that are harmful to humans and other living species. If the tank ruptures, fluid may leak and cause soil or water pollution. Adjacent equipment may be damaged. Unsurprisingly, the consequent financial and reputational costs can be extremely high.
One way to greatly mitigate the risk of a transformer catching fire is to use a high fire point, readily biodegradable dielectric insulating fluid, such as ABB's BIOTEMP.
What is BIOTEMP?
BIOTEMP is a natural ester-based dielectric insulating fluid made from high oleic sunflower or safflower seed oil [2]. Its molecular structure features three long chains of fatty acids attached to a glycerol backbone. It is considered a high oleic natural ester because the oleic fatty acid content is greater than 75 percent of the total composition of the vegetable oil. With a fire point above 300 °C, BIOTEMP is listed as a "less flammable" dielectric fluid by the FM Global insurance company and UL, the independent product safety certification organization. It has a K2 fire hazard classification according to the IEC standard 61100.
By its nature, BIOTEMP is also readily biodegradable. This means that any spill need not be treated as hazardous waste, which saves disposal fees and possible regulatory penalties.
But just how does a fire in a transformer start? And what characteristics of BIOTEMP help thwart it?
Anatomy of a transformer fire
Every fire needs all three elements of the "fire triangle": fuel, oxygen and a source of ignition. In a liquid-filled transformer, the insulating oil can be considered as the fuel. In a sealed transformer there is a limited supply of oxygen dissolved in the oil. In the case of a free-breathing transformer, the oil may become saturated with air. Even in this latter case, where the maximum amount of oxygen (about 30,000 parts per million) may be present in the oil, it is fully dissolved. Consequently, in both cases, there is no "free" oxygen available to support a fire inside the transformer. This underlines the low probability of a transformer actually catching fire.
When a low-impedance fault occurs in a transformer, it creates an electrical arc that may heat up the oil in the immediate vicinity to several thousands of degrees Celsius. Under normal conditions, the transformer's high-current protection will interrupt the fault current and only a small volume of oil in the vicinity of the arc will experience a high temperature. However, if the protection system does not operate properly, then a fire becomes a distinct possibility: The arcing vaporizes the oil and produces a relatively large quantity of combustible gases, some of which will dissolve in the oil, but some of which will escape to the gas space, should one exist.
Depending on the severity of the fault, the increasing gas concentration can cause a pressure buildup large enough to rupture the tank. Then, hot oil and combustible gases will be released, and oxygen-rich air will rush into the tank. If the volume of vaporized liquid present is within the explosive limits of the liquid, and a source of heat is still present, combustion will initiate. At this point, whether the fire builds or self-extinguishes depends primarily on the rate that heat is released from the liquid.
Fire – important risk parameters
There are several different properties of an insulating fluid that determine its propensity to burn. The most important of these are flash point, fire point, auto-ignition temperature and heat release rate. It is also worth noting that it is the flammable vapors from the liquid that serve as fuel – and not the liquid itself.
The flash point is the lowest temperature at which a liquid can vaporize to form an ignitable mixture in air, without necessarily maintaining a fire.
The fire point is the lowest temperature beyond the flash point at which the mixture of vapors and oxygen will burn continuously if ignited. High flash point and high fire point are very desirable.
The auto-ignition temperature is the lowest temperature at which a material will self-ignite and sustain a fire even in the absence of a flame or spark. A liquid whose temperature stays above the fire point will supply a continuous stream of fuel to a fire.
Once a fire has started, the heat release rate determines its ability to propagate. The heat release rate is defined as the amount of thermal energy released per unit of time. A steady or increasing heat release rate will lead to a self-sustaining fire, while a declining heat release rate will result in a fire that self-extinguishes after a period of time.
The vapors produced by different insulating liquids have different concentrations of flammable or explosive elements. There are lower and upper concentrations of these vapors in the air, within which range a mixture would be considered flammable. These are referred to as the upper and lower flammability (or explosive) limits (UFL and LFL, respectively) and the range between these two is referred to as the flammability (or explosive) range. The higher the lower explosive limit is, the larger the volume of vapors needed before a fire can be ignited and then maintained.
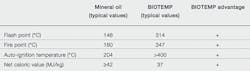
When the key fire risk properties of conventional mineral oil and BIOTEMP are compared, the advantages of BIOTEMP become clear ➔ 1. In an effort to assess these, experiments were conducted to measure the differences in pressure buildup, explosive limits and heat release rate between the two.
1 Fire risk properties
Pressure buildup during arcing
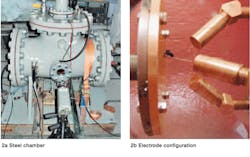
A total of 10 multi-cycle, high-energy (1 to 4 MJ) arcing tests were performed in BIOTEMP and a typical mineral oil by an independent laboratory. The tests were performed in a T-shaped steel chamber, including a 30 to 35 L nitrogen gas cushion ➔ 2. Three rod electrodes with 4 cm electrode gaps were immersed in the fluids.
2 Test setup for high-energy arcing fault experiment
Three-phase AC short-circuit tests at 12 kV and 5.5 kA rms current were carried out with variable short-circuit durations to simulate different arc energies. After each test, about 1 L of gas in the head space and 40 ml of oil were sampled and analyzed.
3 Total gas generation during high-energy arcing fault tests
BIOTEMP had only about 25 to 50 percent as much dissolved combustibles as the mineral oil, but the total amount of gas in the head space was similar, giving comparable pressures ➔ 3. The peak of the measured pressure wave shows a slight increase in BIOTEMP over mineral oil ➔ 4. The conclusion is that the pressure generated by both fluids during the tests is similar.
4 Peak pressure generated during high-energy arcing fault
Explosive limits
The LFL and UFL of BIOTEMP and a typical mineral oil were measured according to the ASTM E 918 standard test method by another independent laboratory. The sample was injected into an appropriately prepared test vessel through a septum, followed by the addition of air up to the required test pressure (14.7 psi). Ignition attempts were made using a high-voltage constant arc (10 kV, 0.25 mA). The concentration of the test mixture was varied between trials until the LFL and UFL for the sample was determined. The results show that an explosive environment will exist if more than 0.6 percent of the volume of an air/fuel mixture is comprised of vaporized mineral oil ➔ 5. For BIOTEMP, this level must be much higher – 9.0 percent – for an explosive environment to exist. Not only that, but, for BIOTEMP, the temperature has to be about 200 °C higher, too, for an explosive environment to exist. This gives BIOTEMP a significant advantage over mineral oil.
5 Explosive limits of BIOTEMP and mineral oil vapors
The explosive nature of mineral oil compared with BIOTEMP was illustrated using oil-filled pole-mounted transformer tanks through which up to 8,000 A flowed for up to three cycles. The lid of the mineral oil setup was blown apart and a fire started after the hot oil was exposed to the atmosphere. In the corresponding test with BIOTEMP, the lid vented and, although a small amount of carbonized oil and vaporized oil spewed out of the tank, there was no fire ➔ 6.
6 High-energy arc fault test
Heat release rate Heat release rate measurements were also made by the same independent laboratory following an adaptation of the ASTM 1354 standard test method. Fluid samples in a ceramic bowl were placed under a heat source delivering 25 kW/m2. When vapors formed, an ignition source above the fluid was triggered until a fire started.
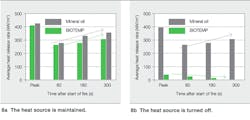
Two test cases were investigated: In one, the setup was maintained after ignition, and, in the other, the heat source was turned off and the sample removed, to simulate a disconnect breaker trip ➔ 7.
7 Heat release rate test set-up
In the first case, it was observed that the average heat release rates for both fluids increased with time. This is consistent with the fire being fed with volatiles due to the heat source keeping the oil temperature above the fire point. In the second case, when the heat source was turned off, it was observed that the peak heat release rate for BIOTEMP (48 kW/m2) is eight times less than that for mineral oil (397 kW/m2) ➔ 8. For mineral oil, the peak heat release rate and the increase of average heat release with time is similar to that of the first case. In contrast, the BIOTEMP sample shows a decrease in heat release rate with time. The BIOTEMP sample actually self-extinguished after a few seconds of combustion.
8 Heat release rate measurements
Several other parameters were also measured during the experiment, such as the time to ignition, the time to flame-out, the peak heat release rate and the smoke production rate ➔ 9. When the heat source is maintained, the time to ignition, a strong indicator of a material's fire resistance, is about 12 times longer for BIOTEMP (724 s) than that for mineral oil (58 s). The smoke, and therefore pollutant, production rate of burning BIOTEMP is less than half that of burning mineral oil. When the heat source is turned off, the time to ignition for BIOTEMP (723 s) is again about 12 times longer than that for mineral oil (57 s). The smoke production from BIOTEMP under this test condition is 31 times less than that from the mineral oil.
9 Heat release rate parameters
Oil futures
The industry drive toward transformers that are ever more fire-resistant under fault conditions is well served by BIOTEMP. Though the chances of a tank rupture are the same as with mineral oil, BIOTEMP demonstrates a much reduced risk of fire initiation and propagation, not to mention outstanding self-extinguishing properties. This makes a BIOTEMP filled transformer one of the safest liquid filled transformers on the market.
George Frimpong
ABB Power Products, Transformers
Raleigh, NC, United States
[email protected]
Stephane Page
ABB Power Products, Transformers
Geneva, Switzerland
[email protected]
Kjell Carrander
ABB Power Products, Transformers
Ludvika, Sweden
[email protected]
Don Cherry
ABB Power Products, Transformers
South Boston, VA, United States
[email protected]
---------------
References
[1] Foata, M. (2008). Power Transformer Fire Risk Assessment. Paper A2.33, CIGRE Symposium, Sydney, Australia.
[2] Oommen, T. V., Claiborne, C. C. (1998). Biodegradable Insulating Fluid from High Oleic Vegetable Oils CIGRÉ, 15–302. Paris, France.