Galvanic Cathodic Protection for Steel Utility Poles
Corrosion of steel structures in electric transmission, distribution, and substation applications has been around for many years, but it’s getting more attention as electric utilities become more aware of the problem. While corrosion can affect any portion of the structure, it is particularly aggressive below grade where it is the most difficult to access. Because of this and other factors, electric utilities are beginning to initiate programs to assess structures for corrosion damage. They are evaluating restoration options and implementing corrosion mitigation to extend the useful service life of these important assets.
Structural Assessment
The structural condition of steel structures is typically determined by measuring structural components and calculating the loss of strength. As structures are evaluated, they can be grouped into specific condition categories for more effective management. Structures in good condition with little or no corrosion can be scheduled for reassessment during subsequent assessment cycles. There are, however, structures needing more immediate attention, which can be focused on for immediate follow-up. Corrective actions can include restoration and application of coatings and cathodic protection.
This system allows for better management and helps estimate budget dollars more accurately for follow up action. By utilizing a recurrent cycle of assessment, mitigation and restoration the reliability of older structures can be greatly improved.
Environmental Assessment
The rate at which steel corrodes below-grade varies significantly based on site conditions, especially soil. Environmental factors at any given site can be evaluated through the collection of soil conditions and characteristics to determine potential corrosion activity.
Primary risk factors in the local soil environment include, but are not limited to, soil resistivity, structure to soil potential, soil pH and REDOX or the oxygen reduction reaction. Other contributing factors can include time of wetness, temperature, land use and soil contaminants (i.e., chlorides)
- The evaluation of this data is used to categorize structures into manageable groups for follow up mitigation. The categories can be defined as follows:
- Mild – Slightly elevated environmental factors that indicate potential for corrosion. Structures are at the threshold where they may require some type of mitigation to prevent the start of corrosion activity.
- Moderate – More serious potential for corrosion activity. Structures should have some type of mitigation applied to offset corrosion deterioration.
- Severe – Factors indicate a severe corrosion risk. Structures should have mitigation applied in the form of coatings at a minimum and be evaluated for cathodic protection.
As environmental site conditions are not static, structures should be reinspected at regular intervals or anytime site conditions change due to nearby construction, agricultural activity, or other contributing factors.
Mitigation
The most practical and effective mitigation efforts include the application of protective coatings. Coatings are the primary means of corrosion protection in most corrosion control systems because they create a barrier on the steel surface to protect against corrosive elements.
Cathodic Protection
Cathodic Protection (CP) is an advanced secondary method of corrosion mitigation often used in conjunction with coatings, but also used separately when conditions dictate.
Through the installation of CP, corrosion activity can be effectively transferred to sacrificial anodes. Through this application, the anodes now corrode in place of the structure. Installation is typically achieved by placing the anode in soil near the structure and bonding it to the structure by a conductive lead wire.
Types of Cathodic Protection
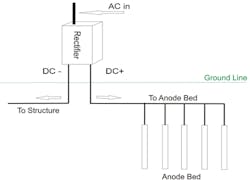
Impressed Current Cathodic Protection (IC-CP) systems are considered an active protection system typically used on large structures, like oil and gas pipelines and other applications where there is need for more protection.
Even though IC-CP is more active and can protect large constructs it does have limitations:
- Cost – on average rectified systems are more costly to purchase, install, and maintain.
- Power – they require a consistent source of power which can be a challenge in remote areas.
- Maintenance – IC-CP systems require frequent periodic inspection and maintenance at regular intervals.
- Age – depending on the manufacturer as rectifier models age their components may no longer be readily available requiring replacement.
Galvanic Cathodic Protection systems protect the structures they are bonded to by means of simple galvanic reaction. Because the anode is higher in electrochemical potential than the steel it therefore has a more “active” potential. The corrosion process is transferred from the steel to the anode. These are considered passive systems because they rely on surrounding site conditions to drive the anode reaction.
- Size – typically used on smaller structures where the CP needs are less.
- Adjustment – performance cannot be adjusted without the addition or removal of anodes.
- Limited protection – greater protection cannot be achieved by simply adding more anodes.
- At some point, the balance of performance vs. cost limits the number of anodes applied.
- Seasonal performance – dependent on-site conditions and may become dysfunctional, if they dry out, especially if they are set shallow.
Sizing and Selection
The type and number of anodes installed at each structure vary based on the exposed surface area of the structure and surrounding soil conditions. The size and length of the anodes also vary and are selected depending on several factors including expected performance, site conditions, and design criteria.
Galvanic anodes come in two different forms; either unpackaged (bare –) or packaged with an engineered backfill (bagged). Each has its benefits and specific uses and can be selected based on application requirements.
Criteria and Monitoring
Regardless of the type of cathodic protection, there are three primary CP industry standards used by the Association for Materials Protection and Performance (AMPP) previously the National Association of Corrosion Engineers (NACE) to ensure protection and performance on buried steel (NACE SP0169). It is not necessary to meet more than one of the following:
- Considering all current and resistance drops and with CP applied, achievement of structure to soil electrochemical potential measurement of equal to or more negative than *-.850 mVcse.
- Assuming no current or resistance drop and with CP applied, achievement of structure to soil electrochemical potential measurement of equal to or more negative than *-.850 mVcse.
- Considering the existing structure potential and with CP applied, achievement of a polarization change or “shift” equal to or more negative than *100 mVcse.
*With reference to a CUSO4; (Copper/Copper Sulfate) reference cell.
Plug and Play: Remote Monitoring Systems
Regular assessment of CP systems is necessary to ensure performance but can be costly and represent challenges when installed in remote, restricted, or difficult access areas.
Once the RMUs are installed, setup can be verified by means of contact with the monitoring service provider so their operations can be verified before leaving the field.
The user account is set up through the monitoring service provider similar to the setup of a new cell phone service. The electric utility customer retrieves their information by logging in to their personalized account and viewing the data via webpage. This information can be downloaded in the form of a Microsoft Excel spreadsheet for viewing and record retention.
The typical download information can be customized to include:
- Site name (structure number)
- Battery status
- Performance (amp output, structure potential, individual anode performance, etc.)
- Security (open door)
- Temperature
Units come in various sizes, capabilities, and program options so it’s important to determine the correct model well in advance of any installation project.
Economics
Electric utilities across the U.S. are struggling to assess their risk on thousands of assets and pressures on operations and maintenance (O&M) budgets seem to grow daily.
While scraping together enough spare O&M budget for a small project may work occasionally, it likely won’t address the long-term financial support of an effective asset management program.
Because steel structures are defined as “long-lived (fixed) assets” which incur “post-acquisition expenditures” during their service life, costs associated with measures are expected to provide a more permanent benefit in longevity, utility, or worth (betterments) that can be capitalized.
To meet the betterment definition under Generally Accepted Accounting Principles (GAAP), the measure must improve the asset in at least one of four ways:
- Increase the asset’s useful life over that which was originally estimated.
- Improve the quality of the asset’s output.
- Increase the quantity of the asset’s output.
- Reduce the costs associated with operating the asset.
The use of capitalized budget dollars on the installation of cathodic protection systems meets the unit of property threshold under the Federal Energy Regulatory Commission (FERC) criteria and resets the appreciation/accounting time cycle on the asset.
Though corrosion deterioration is still a large problem for electric infrastructure, there are many options to address the growing concern.
Galvanic cathodic protection is simple, relatively inexpensive to install, and usually requires low maintenance, while providing reliable corrosion protection for the structures. When integrated into an effective assessment management program, valuable time and resources can be saved for utilities.
With the utilization of these combined technologies, the idea of “plug and play” cathodic protection systems are at hand and may now be more attractive to utilities who have avoided their use in the past.
It is hoped that this information generates further interest and investigation into practical economic corrosion control solutions to help maintain the national power grid through the responsible use of practical economic solutions that improve system reliability and resiliency.
Kevin Niles is a product manager and Corrosion Engineer employed by Osmose Utilities Services, Inc. with over 30 years of experience in the Electric Utility Industry assessing, maintaining, and restoring steel and wood utility structures throughout the US, Canada and Australia. He also has significant experience assessing, restoring and maintaining underground distribution and grounding systems. Kevin is an AMPP (Formerly NACE) Senior Corrosion Technologist and an active committee member with AMPP, IEEE, ASTM, ASCE and ASNT. He is also a current committee officer on the AMPP SC 11 Electric Power standards development committee and IEEE WG 12 Joint Corrosion Committee.
About the Author
Kevin Niles
Kevin Niles is a Product Manager and Corrosion Engineer employed by Osmose Utilities Services, Inc. with over 30 years of experience in the Electric Utility Industry assessing, maintaining, and restoring steel and wood utility structures throughout the US, Canada and Australia. He also has significant experience assessing, restoring and maintaining underground distribution and grounding systems. Kevin is an AMPP (Formerly NACE) Senior Corrosion Technologist and an active committee member with AMPP, IEEE, ASTM, ASCE and ASNT. He is also a current committee officer on the AMPP SC 11 Electric Power standards development committee and IEEE WG 12 Joint Corrosion Committee.